Benzodiazepines: The Basics and Beyond

Of all the psychiatric drugs available, benzodiazepines (BZDs) are among the most widely used. They are approved for a wide variety of indications, and it’s estimated that one in 10 American adults—over 25 million people—have a BZD prescription (www.tinyurl.com/mryyj8y5; Maust DT et al, Psychiatr Serv 2019;70(2):97–106). But, of course, BZDs are associated with a host of well-known adverse effects as well. And long-term use is associated with worse outcomes in conditions like anxiety, PTSD, personality disorders, and substance use disorders. Here, we delve into the essential pharmacology of BZDs, enabling you to better understand their actions, uses, and risks so that you can safely and effectively manage patients taking these medications.
Pharmacology
BZDs produce their effects by enhancing the actions of the neurotransmitter GABA at the GABA-A receptor (Griffin CE et al, Ochsner J 2013;13(2):214–223). Each GABA-A receptor has a single BZD receptor site where drug binding causes a conformational shift (ie, a change in shape), allowing GABA to bind, chloride to flow in, and the cell to hyperpolarize.
Hyperpolarization effectively raises the energy threshold required for neurons to activate, making neurons less likely to fire, which is the primary reason why BZDs have a calming effect. As all BZDs operate through this shared mechanism, they generate similar physiological effects. Yet several factors play into how strongly these effects are felt and how long they last.
Lipophilicity
BZDs are all lipophilic to some degree, which allows them to be readily distributed into the brain and central nervous system (CNS) (Peng L et al, Med Clin North Am 2022;106(1):113–129). The more lipophilic the drug, the faster the onset of action; since alprazolam and diazepam are the most lipophilic, they are the fastest to start working.
Metabolism
Every BZD is metabolized in the liver. For most BZDs, the process begins with oxidation by cytochrome P450 (mainly CYP3A4), followed by a conjugation step known as glucuronidation. The resulting glucuronide metabolite is then excreted in the urine. The length of time that a drug remains in the body is therefore dependent on both the liver and the kidneys, and duration of action might be prolonged in the case of hepatic and renal disease (more on this below). In addition, some BZDs have active metabolites that prolong the clinical effect.
Half-life
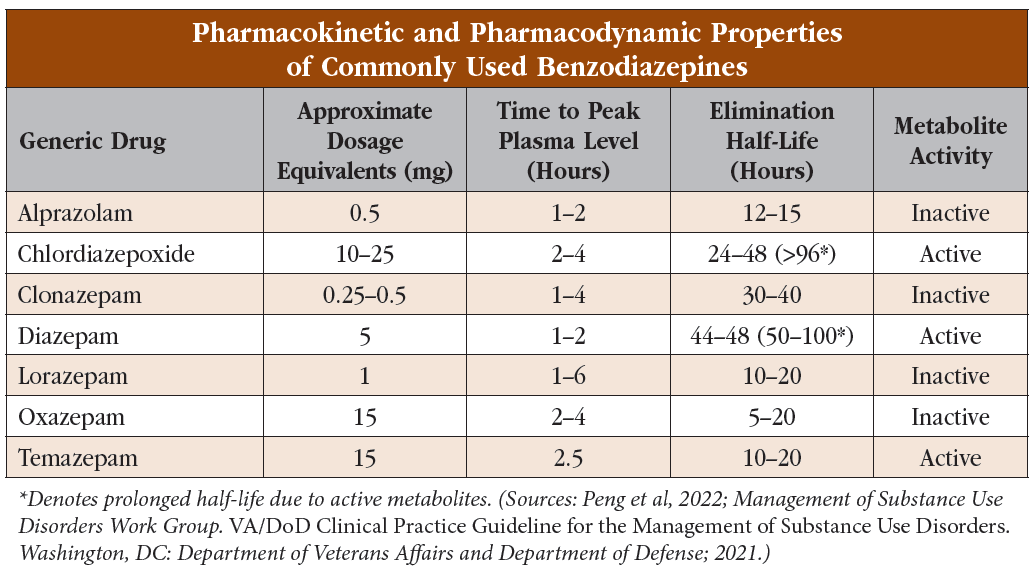 BZDs range widely in terms of their half-life, from a few hours (midazolam) to a few days (diazepam). Drugs with shorter half-lives are more reinforcing and associated with rebound anxiety between doses. They also tend to be more difficult to discontinue, especially toward the end of a drug taper. Of the commonly prescribed BZDs, alprazolam has the shortest half-life, and therefore it can be the most reinforcing and difficult to taper.
BZDs range widely in terms of their half-life, from a few hours (midazolam) to a few days (diazepam). Drugs with shorter half-lives are more reinforcing and associated with rebound anxiety between doses. They also tend to be more difficult to discontinue, especially toward the end of a drug taper. Of the commonly prescribed BZDs, alprazolam has the shortest half-life, and therefore it can be the most reinforcing and difficult to taper.
Potency
BZDs differ in their potencies at the GABA-A receptor (see “Pharmacokinetic and Pharmacodynamic Properties of Commonly Used Benzodiazepines” table). A helpful way to remember dose conversions for some common BZDs is the acronym CAL:
- Clonazepam (0.25–0.5 mg)
- Alprazolam (0.5 mg)
- Lorazepam (1 mg)
Keep in mind that these relative potencies are based on subjective patient feedback, which is an inexact science. Therefore, closely monitor the patient’s response when switching agents to make sure the new dose is neither too high nor too low.
Special considerations
Hepatic and renal impairment
The liver is responsible for metabolizing BZDs, and the kidneys are responsible for eliminating the metabolites. Avoid agents with long half-lives and active metabolites, such as diazepam or chlordiazepoxide, in patients with liver or kidney impairment since the duration of action can become unpredictable and multiple doses can easily accumulate.
During metabolism, a few BZDs skip the initial oxidative step in the liver and instead proceed directly to glucuronidation. This means their metabolism is less dependent on overall liver function, which makes them good choices in patients with liver disease or in older patients (Peng et al, 2022). These drugs also don’t have active metabolites, meaning they are suited for patients with renal disease as well. As a helpful mnemonic to remember these BZDs—temazepam, oxazepam, and lorazepam—think of them as not taking a “TOL” on the liver (see table).
CYP450 interactions
All BZDs, other than “TOL” drugs, undergo CYP450 metabolism, primarily by CYP3A4. Strong CYP3A4 inducers can decrease BZD levels and lead to breakthrough anxiety or withdrawal. Some common strong inducers include:
- Carbamazepine
- Phenobarbital
- Phenytoin
- Rifampin
- St. John’s wort
Strong CYP3A4 inhibitors, such as grapefruit, erythromycin, nefazodone, and some protease inhibitors, can increase serum levels and result in sedation. Avoid these combinations whenever possible, and if they cannot be avoided, monitor your patient carefully (Abernathy DR et al, Curr Med Res Opin 1984;8 Suppl 4:80–93).
Unlike the other BZDs, diazepam is metabolized by both CYP3A4 and CYP2C19. In addition to the CYP3A4 interactions listed above, be cautious with CYP2C19 inhibitors such as fluoxetine, fluvoxamine, cannabidiol, and omeprazole. We always recommend checking for CYP450 interactions with a database such as Micromedex (www.micromedexsolutions.com/micromedex2/librarian/) or Lexicomp (www.wolterskluwer.com/en/solutions/lexicomp).
Other CNS depressants
Unsurprisingly, BZDs can act synergistically with other CNS depressants. BZD prescriptions are associated with increased rates of hospitalization and mortality in patients with alcohol use disorder (Heikkinen M et al, Addiction 2021;116(8):1990–1998). There is also a black box warning regarding BZD and opioid co-prescribing due to increased risk of respiratory depression, sedation, and death (www.tinyurl.com/5e8v8b8u).
Prescribing BZDs to patients using opioids, either prescribed or illicit, is not entirely contraindicated but should be done with extreme caution:
- Only prescribe BZDs after all alternatives have been exhausted
- Keep the duration as short as possible
- Keep the dose as small as possible
Medications for opioid use disorder (OUD) (buprenorphine, methadone) should not be withheld from patients taking BZDs due to the harms associated with untreated OUD (www.tinyurl.com/bde4dbb8). Mitigate risks of co-prescribing through care coordination, careful monitoring, BZD tapering, and provision of naloxone.
Urine toxicology
Interpreting urine screens for BZDs can be confusing. False negatives are common, particularly with three frequently used BZDs—lorazepam, alprazolam, and clonazepam. This is because screening immunoassays detect oxazepam and nordiazepam, which are metabolites of some but not all BZDs (Moeller KE et al, Mayo Clin Proc 2017;92(5):774–796). The metabolites of these drugs sometimes cross-react to give a true positive result, but not always, and the issue can be exacerbated when the doses are low, since urinary metabolite concentration is also low. For more information, see “Urine Drug Screens: What You Need to Know” in CATR May/June 2022.
False positives are uncommon, though they can be seen with sertraline and the anti-inflammatory drug oxaprozin (Moeller et al, 2017; Ferguson M and Mosher K, Pract Pain Manag 2015;15(3)). If you suspect inaccurate results, conduct a confirmatory test using GC-MassSpec. The length of time that BZDs can be detected in urine depends on half-life and can range from three days (lorazepam) to 30 days (diazepam) after last consumption.
Designer BZDs
Designer BZDs structurally resemble approved drugs but don’t have medical uses. They are usually chemical analogs or derivatives of existing BZDs, manufactured to be illicitly sold. Because of their structural similarities, they also act as GABA-A receptor modulators, but their potencies, degrees of lipophilicity, and half-lives are usually poorly understood and unpredictable.
These substances are a growing public health concern (Yu X et al, Expert Rev Clin Pharmacol 2023;16(2):109–117). Because of their chemical alterations, they are not classified under the Controlled Substances Act, making their regulation difficult. Consequently, they are available in retail shops or online, with an increasing number being bought and sold over the dark web (Shapiro AP et al, Psychosomatics 2019;60(6):625–629).
Most designer BZDs aren’t detectable via standard urine drug screens. As such, it’s critical to look for signs of BZD intoxication or withdrawal, even if the patient’s urine is negative for BZDs. Be sure to ask your patients about their use of designer BZDs or other substances obtained from non-medical sources.
CARLAT VERDICT
A thorough understanding of the pharmalogic properties of the various BZDs is essential for proper management. Whether you are starting or discontinuing a BZD, you should consider the medication's lipophilicity, half-life, and potency, as well as patient characteristics such as renal and hepatic function, and other medications that they are taking.


_-The-Breakthrough-Antipsychotic-That-Could-Change-Everything.webp?t=1729528747)



