Treating Opioid Use Disorder During Pregnancy
Opioid use during pregnancy has skyrocketed over the past decade with opioid use disorder (OUD) diagnoses at childbirth more than doubling from 2010 to 2017 (Hirai AH et al, JAMA 2021;325:146–155). Untreated OUD is associated with a host of negative outcomes for both parent and child, though many of these can be mitigated with medications for opioid use disorder (MOUD), namely methadone and buprenorphine. However, pharmacokinetic shifts that occur during pregnancy affect how these medications should be prescribed. Here, we’ll review the evidence supporting MOUD during pregnancy and get into the nuts and bolts of how to properly prescribe them.
Why treat?
Untreated OUD in the perinatal period is associated with maternal cardiac arrest, intrauterine growth restriction, placental abruption, premature birth, and low birth weight (Nørgaard M et al, Subst Abuse 2015;9(Suppl 2):5–11). Additionally, opioid overdoses have emerged as a primary driver of postpartum maternal mortality (Bruzelius E et al, JAMA 2022;328(21):2159–2161). Opioid withdrawal can be especially dangerous during pregnancy. The catecholamine surge precipitated by withdrawal induces uterine contractions, diminishing placental blood flow and oxygen supply, which can lead to fetal hypoxia, preterm labor, and even fetal demise (Miller CB and Wright T, Acad Forensic Pathol 2018;8(4):865–873).
Effective treatment of OUD during pregnancy, particularly with buprenorphine and methadone, can significantly mitigate these adverse outcomes. Minimizing exposure to illicit opioids not only reduces the risk of overdose, but also is associated with healthier infant birth weight, lower likelihood of premature birth, and decreased intensity of neonatal opioid withdrawal syndrome (NOWS). Moreover, the improved psychosocial stability that comes with treatment decreases the risk of communicable diseases and comorbid substance use, improves maternal nutrition, and encourages engagement in perinatal care (Jones HE et al, J Subst Abuse Treat 2008;35(3):245–259).
Patients may ask about medically assisted withdrawal, commonly called medication-assisted “detox,” which involves a short taper of buprenorphine or methadone with the goal of ultimately stopping all medication and opioid use. The use of medication is intended to result in fewer opioid withdrawal symptoms compared to stopping opioid agonists cold turkey. While the approach is probably safe for the fetus, it rarely results in sustained abstinence and is associated with high rates of return to illicit opioid use (Terplan M et al, Obstet Gynecol 2018;131(5):803–814).
Choosing the right MOUD
Buprenorphine and methadone are both safe and effective in pregnancy. An advantage of buprenorphine is that it results in less severe NOWS. In a landmark study, infants exposed to buprenorphine required 89% less morphine, had a 58% reduction in treatment duration, and had a 43% reduction in length of hospital stay compared to infants exposed to methadone (Jones HE et al, N Engl J Med 2010;363(24):2320–2331). Infants exposed to methadone also have been found to have modestly increased rates of preterm birth, small size for gestational age, and low birth weight relative to those exposed to buprenorphine (Suarez EA et al, N Engl J Med 2022;387(22):2033–2044).
On the other hand, methadone may be better at retaining patients in treatment over the course of pregnancy. In the study referenced above, pregnant patients on buprenorphine were nearly twice as likely to discontinue MOUD compared to those taking methadone: 33% vs 18% (Jones et al, 2010). Like much of medicine, methadone and buprenorphine have pros and cons. Therefore, medication choice should come from shared decision making, taking patient preference, prior response, and treatment availability into account (Guille C et al, Psychiatr Res Clin Pract 2019;1(1):27–31).
Special considerations during pregnancy
Physiologic shifts
Many physiologic shifts occur during pregnancy that affect the pharmacokinetics of buprenorphine and methadone, including:
- Increases in volume expansion and cardiac output
- Decreases in protein binding and drug absorption
These changes tend to peak during the second trimester and persist until delivery (Kazma JM et al, J Pharmacokinet Pharmacodyn 2020;47(4):271–285).
Drug metabolism
Drug metabolism increases significantly during pregnancy as well. Surges in progesterone and estrogen trigger the induction of cytochrome P450, leading to reduced half-life and lower peak plasma concentrations of methadone and buprenorphine (McCarthy JJ et al, J Addict Med 2018;12(3):241–246). Genetic variations in CYP450 polymorphisms lead to ultrarapid methadone metabolism in up to 44% of pregnant patients, reducing the drug’s half-life even further—from up to 59 hours in non-pregnant patients to eight hours in pregnant patients and a mere four to six hours in ultrarapid metabolizers (Badhan RKS and Gittins R, J Subst Abuse Treat 2021;130:108521).
Buprenorphine has three active metabolites, unlike methadone, which only has a single inactive metabolite (Brown SM et al, Anesthesiology 2011;115(6):1251–1260). These active metabolites might serve as a protective buffer against the increased metabolism, leading to buprenorphine typically needing less active adjustment during pregnancy than methadone.
Prescribing MOUD
 Protocols for starting MOUD in pregnancy are similar to those in the general population, but with the special consideration of minimizing maternal withdrawal given the potential for adverse fetal effects. Whichever medication you and your patient choose, it should be started as soon as possible and continued after delivery.
Protocols for starting MOUD in pregnancy are similar to those in the general population, but with the special consideration of minimizing maternal withdrawal given the potential for adverse fetal effects. Whichever medication you and your patient choose, it should be started as soon as possible and continued after delivery.
Buprenorphine
Pregnant individuals under 24 weeks of gestation can usually undergo outpatient buprenorphine induction. Refer your patient to the emergency department if they experience precipitated withdrawal or if their Clinical Opiate Withdrawal Scale (COWS) score goes over 14. Hospitalization is recommended for buprenorphine induction for all patients after 24 weeks of gestation, which is the gestational age at which fetal monitoring can be done efficiently.
The induction procedure is essentially the same as in non-pregnant patients (see CATR Nov/Dec 2021):
- Give an initial dose of 2–4 mg once COWS >8
- An additional 2–4 mg can be given every two to four hours for a total of 8–12 mg in the first 24 hours
- The dose can be increased by 8 mg each day, up to a total of 24 mg daily
Buprenorphine doses usually do not need to be increased as much as methadone during pregnancy, though some patients may benefit from slightly higher or more frequent doses; adjust according to your patient’s symptoms (Young JL and Martin PR, Psychiatr Clin North Am 2012;35(2):441–460).
Recent evidence has found no adverse effects in pregnancy with buprenorphine/naloxone (Link HM et al, Am J Obstet Gynecol MFM 2020;2(3):100179). The monoproduct and combination product are both regularly used, so choose whichever is accessible and preferred by your patient.
Methadone
Methadone treatment can be initiated as an outpatient early in pregnancy, typically in the first or early second trimester. Later in pregnancy, methadone is usually started in an inpatient setting, which allows the patient to be in a controlled environment as the medication is titrated and facilitates fetal monitoring.
If the patient presents without symptoms of withdrawal:
- Give a small initial dose of 5–10 mg
- Follow with an additional dose if withdrawal develops
If the patient presents in moderate or severe withdrawal:
- Give an initial 20–30 mg dose
- Provide an additional 5–10 mg every three to six hours, until withdrawal symptoms are relieved or a maximum of 50 mg is reached
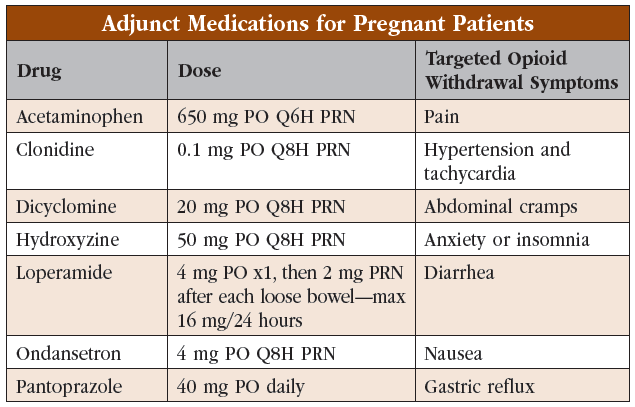
Additional symptomatic treatment can be given for any residual symptoms that are present after the maximum methadone dose is reached. Other than avoiding nonsteroidal anti-inflammatory drugs, medication choice and dosing are similar to treating non-pregnant patients. For a quick review of symptomatic treatments, see the “Adjunct Medications for Pregnant Patients” table.
As long as the patient remains in the hospital, on day 2:
- Give the total dose for the previous 24 hours as a single morning dose
- Give a supplement of 5–10 mg later in the day if withdrawal or cravings develop
Repeat this pattern, with small daily increases until the patient is no longer experiencing withdrawal or cravings. Once this maintenance dose is reached, the patient can continue methadone as an outpatient.
Once discharged, adjust doses more slowly—typically 5–10 mg per week. Daily doses commonly exceed 120 mg; in fact, higher doses are associated with better outcomes (McCarthy JJ et al, Am J Obstet Gynecol 2005;193(3):606). When possible, methadone should be given twice daily, which leads to steadier plasma concentrations.
Postpartum
Patients should remain on MOUD following delivery; overdose risk peaks six months postpartum and is nearly 10-fold higher for those not on MOUD (Schiff DM et al, Obstet Gynecol 2018; 132(2):466–474). As the body transitions back to its pre-pregnancy physiology, some patients, particularly those on methadone, may need their dose decreased. However, the total reduction required might be minimal, and some patients may find no need for dose adjustment at all (Pace CA et al, J Subst Abuse Treat 2014;47(3):229–232). Follow your patient closely and adjust the dose based on your clinical assessment.
Both buprenorphine and methadone pass into breast milk and have a beneficial effect on NOWS. MOUD have been shown to decrease incidence of NOWS, shorten hospitalization, and improve mother-infant bonding (Bagley SM et al, Addict Sci Clin Pract 2014;9(1):19). Given these advantages, encourage breastfeeding for patients on MOUD who abstain from illicit substances and are not HIV positive (Reece-Stremtan S, Breastfeed Med 2015;10(3):135–141).
CARLAT VERDICT
The past decade has seen a dramatic increase in the prevalence of OUD in pregnancy and a concomitant rise in adverse events. Buprenorphine and methadone are safe and effective during pregnancy, and their use is associated with a host of benefits. Medication choice and proper dosing involve understanding the physiological changes of pregnancy and an exploration of individual patient needs.




_-The-Breakthrough-Antipsychotic-That-Could-Change-Everything.webp?t=1729528747)



