Auvelity: A Fast-Acting Antidepressant?
Chris Aiken, MD. Editor-in-Chief of The Carlat Psychiatry Report. Assistant Professor, NYU Langone Department of Psychiatry. Practicing psychiatrist, Winston-Salem, NC.
Dr. Aiken has no financial relationships with companies related to this material.
Auvelity (pronounced “aw-VEHL-ah-tee”) brings to mind “augment with velocity,” and that is the bold claim for this new antidepressant. Auvelity is a combination of two older medications: bupropion (circa 1985) and dextromethorphan (circa 1958), a cough suppressant found in Robitussin DM.
How it works
Like ketamine, dextromethorphan (DM) increases glutamate transmission through NMDA antagonism. This makes Auvelity the first oral antidepressant to move beyond the traditional monoamines of serotonin, norepinephrine, and dopamine. This glutamatergic mechanism is shared by several third-line interventions for treatment-resistant depression (amantadine, d-cycloserine, ketamine, lamotrigine, and minocycline). DM also has anticonvulsant and neuroprotective effects, and blocks serotonin and dopamine reuptake, complementing bupropion’s effects on norepinephrine and dopamine.
The pairing with bupropion also accomplishes a pharmacokinetic goal by increasing DM’s half-life. Bupropion inhibits DM’s major metabolic pathway (CYP2D6), extending its half-life from four hours to 22 hours. A similar effect is achieved with Nuedexta, another branded coformulation of DM that is FDA approved in pseudobulbar affect, a neurologic disorder characterized by sudden, uncontrollable bouts of laughter or tears. Nuedexta pairs DM with quinidine, a strong CYP2D6 inhibitor that otherwise adds nothing to Nuedexta’s therapeutic effects.
Clinical trials
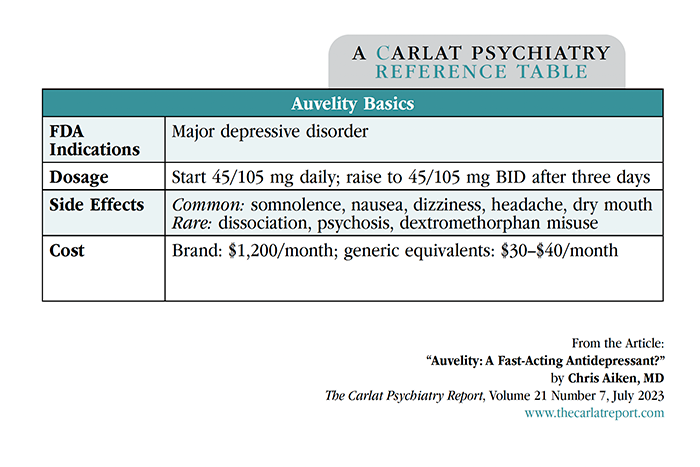
Compared to bupropion, clinical trials show that Auvelity brings greater speed and efficacy to the treatment of depression. One was large (n=327) and one small (n=80). The large trial confirmed that the combo worked better than placebo, which is not a surprise considering it contains a therapeutic dose of bupropion. What did stand out was the speed of onset, with separation from placebo in the first week compared to weeks two to four for other antidepressant trials (Iosifescu DV et al, J Clin Psychiatry 2022;83(4):21m14345). The small trial confirmed the speed-of-onset data and added new information by comparing the combination to bupropion monotherapy. Auvelity was more effective than bupropion alone, with remission rates of 47% vs 16% after six weeks (Tabuteau H et al, Am J Psychiatry 2022;179(7):490–499). However, it is unclear whether Auvelity works in treatment-resistant cases. The manufacturer conducted a large trial in treatment-resistant depression with negative results, although the details are unpublished.
DM has sedative properties that contributed to a higher rate of side effects with the combination pill. One in 16 patients stopped the medication due to side effects, most often somnolence, nausea, dizziness, headache, and dry mouth. No cases of psychosis, dissociation, serotonin syndrome, or addictive behaviors were seen with Auvelity, although these are potential problems with DM and have been reported in earlier investigations. Whether DM can precipitate mania is unknown, but small studies in bipolar depression (which paired DM with quinidine, not bupropion) found either no manic switching or improvement in manic symptoms (Lee SY et al, Int J Bipolar Disord 2020;8(1):11).
Studies of Auvelity are underway in nicotine cessation and agitation in dementia.
Controversies
Potential for misuse
Although Auvelity is not classified as a controlled substance, DM has a recognized misuse potential as “poor man’s PCP” or “robotripping.” There was no evidence of inappropriate use in the clinical trials, in which DM was dosed far below the abusable level (90 mg/day). However, the interaction with bupropion is likely to push DM’s serum levels into the abusable range. Based on the pharmacokinetic data we requested from the manufacturer, bupropion raises peak levels of DM 40-fold and the total exposure to the drug (area under the curve) 60-fold (www.tinyurl.com/mr37cf8y). Furthermore, patients with a recent history of substance use disorders were excluded from the trials.
Generic substitution
Another area of controversy is generic substitution. Auvelity comes as a tablet containing 45 mg of DM and 105 mg of bupropion. The recommended target dose is 45/105 mg twice daily, with a steep out-of-pocket cost of $1,200/month. There are various potential generic workarounds that would make the drug affordable. DM is available by prescription or as a low-cost, over-the-counter generic syrup, though it is actually kept “behind the counter” to limit diversion and misuse. The liquid can be given in the same 45 mg BID dose that is contained in Auvelity (for most DM products, 7.5 mL = 45 mg at a monthly cost of $20). Bupropion is available generically in doses very close to Auvelity’s 105 mg BID (eg, bupropion IR 100 mg BID or bupropion SR 200 mg). Thus, you can prescribe these separate “ingredients” of Auvelity to your patients and save them thousands of dollars, with presumably the same beneficial effects.
Combining DM with other antidepressants
Bupropion’s mechanism of action is unique, and it is not known if DM augmentation will work with other antidepressants. Duloxetine, fluoxetine, and paroxetine provide even stronger CYP2D6 inhibition, but they also carry a risk of serotonin syndrome when combined with DM, itself a serotonin reuptake inhibitor.
How to use
Auvelity’s speed of onset makes it ideal for patients who are hospitalized or otherwise in need of rapid relief from depression. If the rapid titration schedule used in the trials (see “Auvelity Basics” table) is not tolerable, DM could be added in as a lower dose in the evening and raised toward 45 mg BID as tolerated.
Many relevant questions are left unanswered by these studies. Can bupropion be started as monotherapy and DM added later if there is no response? Will raising the bupropion or DM dose help if the patient does not recover? DM was dosed up to 60 mg BID (in combination with bupropion or quinidine) in older trials conducted before industry involvement. These studies tested DM in both unipolar and bipolar depression, but they were small and yielded mixed results that make it difficult to draw conclusions. Perhaps the most important question is whether DM can be safely tapered off after a sustained recovery. Compared to other fields of medicine, we have not done a good job of separating acute from prophylactic therapies. Industry-sponsored trials are not likely to clarify these distinctions. Based on what we know from other augmentation trials, I would recommend waiting at least six months before considering a DM taper, and tapering slowly over at least one or two months. Patients may also need to raise bupropion to the usual dose of 300 mg/day.
CARLAT VERDICT
Auvelity gets a thumbs-up for speed and efficacy, but misses the mark in treatment-resistant depression. Generic substitution gets a green light.

Newsletters
Please see our Terms and Conditions, Privacy Policy, Subscription Agreement, Use of Cookies, and Hardware/Software Requirements to view our website.
© 2025 Carlat Publishing, LLC and Affiliates, All Rights Reserved.


_-The-Breakthrough-Antipsychotic-That-Could-Change-Everything.jpg?1729528747)



