Home » Namzaric and Other Cognitive Enhancers for Dementia
Namzaric and Other Cognitive Enhancers for Dementia
March 1, 2015
From The Carlat Psychiatry Report
Daniel Carlat, MD
Editor-in-Chief, Publisher, The Carlat Report.
Dr. Carlat has disclosed that he has no relevant relationships or financial interests in any commercial company pertaining to this educational activity.
There’s a new medication on the market for the treatment of dementia—the first to come along in several years.
In December of 2014, Actavis and Adamas Pharmaceuticals announced the approval of Namzaric, which is a combination of Namenda XR and Aricept, for moderate to severe dementia. While no one would claim that this combination of two existing drugs constitutes a breakthrough in dementia treatment, it may pose some advantages nonetheless.
Before the introduction of Namzaric, the last truly new medication, memantine (Namenda), was approved in 2003. Before that, we had the approvals of donepezil (Aricept) in 1996, rivastigmine (Exelon) in 2000, and galantamine (Razadyne) in 2001. What followed have been old medications in a new format (Exelon Patch in 2001, Aricept ODT in 2004, Razadyne ER in 2004, Namenda XR in 2010, and high dose Aricept in 2010).
A Review of the Old and New
In this article we’ll take a look at the Namzaric data and come up with some recommendations. But first let’s do a quick review of the evidence for the meds that are already available.
We organize the meds by stage of illness (mild to moderate vs. moderate to severe), because this is how the US Food and Drug Administration (FDA) approves them. However, real patients don’t easily fit into such categories, and real psychiatrists will use their best judgment regarding prescriptions. That often means going off-label—for example, prescribing cognitive enhancers for non-Alzheimer’s dementias, or prescribing Namenda for patients who may not quite meet formal criteria for “moderate to severe” dementia. Use the “my grandmother” rule to guide your decisions—if this patient were your grandmother (or other loved one), what would you do? Because there are so few treatments for dementia, you’d likely stray beyond FDA guidelines in the quest for a good result.
Mild to Moderate Dementia
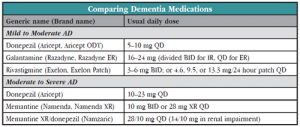
Four different acetylcholinesterase inhibitors (AChEIs) have been approved for mild to moderate dementia: Tacrine (remember Cognex?) was the first but is no longer used due to liver toxicity; then came Aricept, and later Aricept ODT; Exelon and Exelon Patch; and Razadyne and Razadyne ER.
To test the effectiveness of any drug, you have to define an outcome of interest and see if the drug improves it relative to placebo. Most studies of Alzheimer’s disease (AD) rely on the Alzheimer’s Disease Assessment Scale Cognitive Subscale (ADAS-Cog), a 70-point scale that tests memory, language, orientation, and praxis (ability to carry out simple motor skills). Scores range from 0 to 70—the higher the score, the worse the impairment. Patients with mild to moderate dementia typically have ADAS-Cog scores between 15 and 55.
How well do AChEIs work? First of all, these drugs rarely actually improve cognition—instead, they act by reducing the rate of cognitive worsening. Without medications, an average patient with mild AD may experience an increase (worsening) of 5 ADAS-Cog points in a year. AChEIs slow this rise by 2 to 3 points relative to placebo (this is confusingly reported in article abstracts as a “decline” in the ADAS-Cog, when what is meant is a diminution in the rise of the ADAS-Cog as compared to placebo).
What does a 2 to 3 point difference mean clinically? According to one study, a minimum of a 3 point difference is required before a clinician notices that there is a meaningful improvement (Schrag A et al, J Neurol Neurosurg Psychiatry 2012;83:171–173). A 3 point difference might translate to, for example, a patient remembering who came to dinner the night before or being able to dress himself (Boustani M et al, Ann Intern Med 2003;138(11):927–937). The bottom line is that AChEIs beat placebo by a small amount—statistically significant but not always clinically significant.
Moderate to Severe Dementia
Three medications have been approved for moderate to severe dementia: Namenda (along with its patent-extending offspring, Namenda XR), Aricept 23 mg, and Namzaric, the Namenda/Aricept combo product.
Namenda, Namenda XR. Namenda (approved in 2003) and Namenda XR (2010) are N-methyl-D-aspartate (NMDA) antagonists that are both approved for moderate to severe AD based on six-month clinical trials showing small but statistically significant benefit over placebo (Kurz A & Grimmer T, Expert Opin Pharmacother 2014;15(13):1955–1960). As with AChEIs, Namenda will neither reverse nor halt the cognitive decline but may slow it down. It’s pretty well tolerated, with headache, dizziness, and dose-related somnolence most commonly reported.
Its manufacturer Forest Laboratories’ marketing pitch for Namenda XR is that once a day dosing increases convenience and compliance. The company initially announced that it would be discontinuing regular Namenda (available in 5 mg and 10 mg tablets), forcing patients to switch to Namenda XR (available in 7 mg, 14 mg, 21 mg, and 28 mg) if they want to keep taking it. The reasons are economic: old Namenda’s patent is expiring, and the company wants to get patients on their new branded version before generic memantine hits the market. The tactic (called the “forced switch”) has been successfully used by other companies in the past, for better or worse. The New York State attorney general is suing the company for violating anti-trust laws, so for now, the company is continuing to make the IR available under the terms of a court order.
Aricept 23 mg. In 2010, just before the original Aricept went off patent, the FDA approved higher dose Aricept for moderate to severe dementia—at an odd dose of 23 mg. It was a controversial approval (for the fascinating inside story, see Schwartz LM & Woloshin S, BMJ 2012;344:e1086).
The pivotal study randomly assigned 1,400 patients with moderate to severe dementia to either Aricept 23 mg per day or Aricept 10 mg per day. Mindful that a dementia drug can beat its competitor on a numerical cognitive scale without yielding true clinical benefit, the FDA told Eisai (the manufacturer) that its drug would have to show superiority on two scales—a cognitive scale and global functioning scale. After six months, the results were tallied, and 23 mg beat 10 mg on only one of the measures—by a mere 2.2 points out of a 100 point severe symptom scale. Furthermore, the higher dose caused significantly more side effects than the lower dose, particularly nausea (12% vs. 3%) and vomiting (9% vs. 2.5%). Because of these issues, the FDA review team recommended non-approval, but that decision was overridden by the director of the FDA’s neurology division.
Namzaric (Namenda plus Aricept). Now we’ve circled back to Namzaric, which received FDA approval in 2014. Namzaric is a combination of Namenda XR and Aricept (available in two doses: 28mg/10 mg and 14mg/10 mg, Namenda XR/Aricept). The rationale for combining these two medications is that since each one is somewhat effective for moderate to severe dementia and since they have different mechanisms of action, combining them should be even more effective.
In the pivotal Namzaric study, 677 outpatients with moderate to severe AD, who were already stable on an AChEI (mostly Aricept), were randomized to 24 weeks of add-on Namenda XR 28 mg or add-on placebo (Grossberg GT et al, CNS Drugs 2013;27(6):469–478). Patients on the active combination did better than those on an AChEI plus placebo, with higher cognition scores but no difference on activities of daily living scores.
Dr. Carlat's Verdict: For mild to moderate AD, start with an AChEI—we have the most data and experience with Aricept but the others are equally effective. You want to titrate Aricept to an effective dose, which according to FDA guidelines maxes out at 10 mg/day, but patients may tolerate 15 mg to 20 mg a day (Doody RS et al, Drugs Aging 2008;25(2):163–174). For moderate to severe AD, start with Namenda IR, reserving the more expensive Namenda XR for those rare situations in which IR is poorly tolerated. Or use high dose Aricept (either combining two 10 mgs or using the 23 mg version). A more common approach among experts is to stabilize patients on an AChEI, then add Namenda, regardless of whether the dementia is mild, moderate, or severe. For patients who have trouble taking two pills, consider Namzaric. Regardless of what you use, remind patients and their caregivers that these medications do not reverse or stop the decline associated with AD—they just slow it down a little.
General PsychiatryIn December of 2014, Actavis and Adamas Pharmaceuticals announced the approval of Namzaric, which is a combination of Namenda XR and Aricept, for moderate to severe dementia. While no one would claim that this combination of two existing drugs constitutes a breakthrough in dementia treatment, it may pose some advantages nonetheless.
Before the introduction of Namzaric, the last truly new medication, memantine (Namenda), was approved in 2003. Before that, we had the approvals of donepezil (Aricept) in 1996, rivastigmine (Exelon) in 2000, and galantamine (Razadyne) in 2001. What followed have been old medications in a new format (Exelon Patch in 2001, Aricept ODT in 2004, Razadyne ER in 2004, Namenda XR in 2010, and high dose Aricept in 2010).
A Review of the Old and New
In this article we’ll take a look at the Namzaric data and come up with some recommendations. But first let’s do a quick review of the evidence for the meds that are already available.
We organize the meds by stage of illness (mild to moderate vs. moderate to severe), because this is how the US Food and Drug Administration (FDA) approves them. However, real patients don’t easily fit into such categories, and real psychiatrists will use their best judgment regarding prescriptions. That often means going off-label—for example, prescribing cognitive enhancers for non-Alzheimer’s dementias, or prescribing Namenda for patients who may not quite meet formal criteria for “moderate to severe” dementia. Use the “my grandmother” rule to guide your decisions—if this patient were your grandmother (or other loved one), what would you do? Because there are so few treatments for dementia, you’d likely stray beyond FDA guidelines in the quest for a good result.
Mild to Moderate Dementia
Four different acetylcholinesterase inhibitors (AChEIs) have been approved for mild to moderate dementia: Tacrine (remember Cognex?) was the first but is no longer used due to liver toxicity; then came Aricept, and later Aricept ODT; Exelon and Exelon Patch; and Razadyne and Razadyne ER.
To test the effectiveness of any drug, you have to define an outcome of interest and see if the drug improves it relative to placebo. Most studies of Alzheimer’s disease (AD) rely on the Alzheimer’s Disease Assessment Scale Cognitive Subscale (ADAS-Cog), a 70-point scale that tests memory, language, orientation, and praxis (ability to carry out simple motor skills). Scores range from 0 to 70—the higher the score, the worse the impairment. Patients with mild to moderate dementia typically have ADAS-Cog scores between 15 and 55.
How well do AChEIs work? First of all, these drugs rarely actually improve cognition—instead, they act by reducing the rate of cognitive worsening. Without medications, an average patient with mild AD may experience an increase (worsening) of 5 ADAS-Cog points in a year. AChEIs slow this rise by 2 to 3 points relative to placebo (this is confusingly reported in article abstracts as a “decline” in the ADAS-Cog, when what is meant is a diminution in the rise of the ADAS-Cog as compared to placebo).
What does a 2 to 3 point difference mean clinically? According to one study, a minimum of a 3 point difference is required before a clinician notices that there is a meaningful improvement (Schrag A et al, J Neurol Neurosurg Psychiatry 2012;83:171–173). A 3 point difference might translate to, for example, a patient remembering who came to dinner the night before or being able to dress himself (Boustani M et al, Ann Intern Med 2003;138(11):927–937). The bottom line is that AChEIs beat placebo by a small amount—statistically significant but not always clinically significant.
Moderate to Severe Dementia
Three medications have been approved for moderate to severe dementia: Namenda (along with its patent-extending offspring, Namenda XR), Aricept 23 mg, and Namzaric, the Namenda/Aricept combo product.
Namenda, Namenda XR. Namenda (approved in 2003) and Namenda XR (2010) are N-methyl-D-aspartate (NMDA) antagonists that are both approved for moderate to severe AD based on six-month clinical trials showing small but statistically significant benefit over placebo (Kurz A & Grimmer T, Expert Opin Pharmacother 2014;15(13):1955–1960). As with AChEIs, Namenda will neither reverse nor halt the cognitive decline but may slow it down. It’s pretty well tolerated, with headache, dizziness, and dose-related somnolence most commonly reported.
Its manufacturer Forest Laboratories’ marketing pitch for Namenda XR is that once a day dosing increases convenience and compliance. The company initially announced that it would be discontinuing regular Namenda (available in 5 mg and 10 mg tablets), forcing patients to switch to Namenda XR (available in 7 mg, 14 mg, 21 mg, and 28 mg) if they want to keep taking it. The reasons are economic: old Namenda’s patent is expiring, and the company wants to get patients on their new branded version before generic memantine hits the market. The tactic (called the “forced switch”) has been successfully used by other companies in the past, for better or worse. The New York State attorney general is suing the company for violating anti-trust laws, so for now, the company is continuing to make the IR available under the terms of a court order.
Aricept 23 mg. In 2010, just before the original Aricept went off patent, the FDA approved higher dose Aricept for moderate to severe dementia—at an odd dose of 23 mg. It was a controversial approval (for the fascinating inside story, see Schwartz LM & Woloshin S, BMJ 2012;344:e1086).
The pivotal study randomly assigned 1,400 patients with moderate to severe dementia to either Aricept 23 mg per day or Aricept 10 mg per day. Mindful that a dementia drug can beat its competitor on a numerical cognitive scale without yielding true clinical benefit, the FDA told Eisai (the manufacturer) that its drug would have to show superiority on two scales—a cognitive scale and global functioning scale. After six months, the results were tallied, and 23 mg beat 10 mg on only one of the measures—by a mere 2.2 points out of a 100 point severe symptom scale. Furthermore, the higher dose caused significantly more side effects than the lower dose, particularly nausea (12% vs. 3%) and vomiting (9% vs. 2.5%). Because of these issues, the FDA review team recommended non-approval, but that decision was overridden by the director of the FDA’s neurology division.
Namzaric (Namenda plus Aricept). Now we’ve circled back to Namzaric, which received FDA approval in 2014. Namzaric is a combination of Namenda XR and Aricept (available in two doses: 28mg/10 mg and 14mg/10 mg, Namenda XR/Aricept). The rationale for combining these two medications is that since each one is somewhat effective for moderate to severe dementia and since they have different mechanisms of action, combining them should be even more effective.
In the pivotal Namzaric study, 677 outpatients with moderate to severe AD, who were already stable on an AChEI (mostly Aricept), were randomized to 24 weeks of add-on Namenda XR 28 mg or add-on placebo (Grossberg GT et al, CNS Drugs 2013;27(6):469–478). Patients on the active combination did better than those on an AChEI plus placebo, with higher cognition scores but no difference on activities of daily living scores.
Dr. Carlat's Verdict: For mild to moderate AD, start with an AChEI—we have the most data and experience with Aricept but the others are equally effective. You want to titrate Aricept to an effective dose, which according to FDA guidelines maxes out at 10 mg/day, but patients may tolerate 15 mg to 20 mg a day (Doody RS et al, Drugs Aging 2008;25(2):163–174). For moderate to severe AD, start with Namenda IR, reserving the more expensive Namenda XR for those rare situations in which IR is poorly tolerated. Or use high dose Aricept (either combining two 10 mgs or using the 23 mg version). A more common approach among experts is to stabilize patients on an AChEI, then add Namenda, regardless of whether the dementia is mild, moderate, or severe. For patients who have trouble taking two pills, consider Namzaric. Regardless of what you use, remind patients and their caregivers that these medications do not reverse or stop the decline associated with AD—they just slow it down a little.
KEYWORDS dementia

Issue Date: March 1, 2015
Table Of Contents
Recommended
Newsletters
Please see our Terms and Conditions, Privacy Policy, Subscription Agreement, Use of Cookies, and Hardware/Software Requirements to view our website.
© 2025 Carlat Publishing, LLC and Affiliates, All Rights Reserved.


_-The-Breakthrough-Antipsychotic-That-Could-Change-Everything.webp?t=1729528747)



