Vyvanse: A Look at America’s Most Prescribed Stimulant
Why has Vyvanse become by far the most prescribed stimulant in the United States? Great marketing? A great product? Some combination of the two? And more to the point, should you continue to choose it over its much cheaper competitors? Read on for our take on the Vyvanse phenomenon.
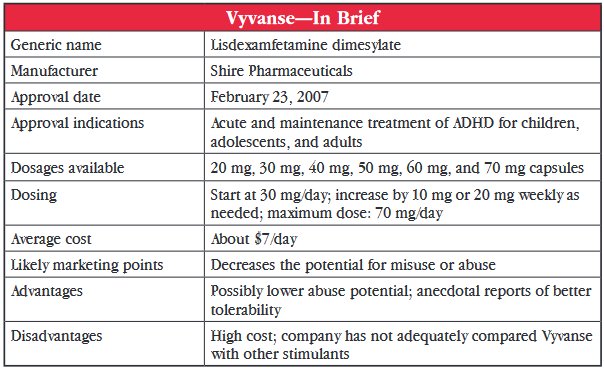
Vyvanse (lisdexamfetamine) was first approved by the US Food and Drug Administration (FDA) for pediatric ADHD in 2007. Later, it was approved for both adults (2008) and adolescents aged 13 to 17 (2010). The medication quickly became a go-to medication for prescribers. In 2013, it was the eighth most prescribed drug of any kind in the US, with over 10.5 million prescriptions and total sales of $1.7 billion—far outpacing its closest stimulant competitor, Focalin XR, which came in at number 44 on the list of most prescribed drugs with just over three million scripts.
How it Works
Vyvanse is lisdexamfetamine, which is dextroamphetamine (the molecular name of Dexedrine), bound to a lysine molecule. It remains inactive until hydrolyzing enzymes cleave off the lysine and convert it to the active dextroamphetamine. The manufacturer’s claim is that this gives the drug a lower potential for abuse because the active ingredient is only released when the medication is swallowed, making it inactive if snorted or injected. Interestingly, there are numerous web sites instructing would-be amateur chemists on how to perform the hydrolysis reaction at home prior to ingestion in order to have access to the pure dextroamphetamine form (see, for example, http://bit.ly/1yiUFDt).
Vyvanse was approved in each age group based on four-week studies comparing fixed doses of 30, 50, and 70 mg/day to placebo. In addition, a maintenance indication in adults was approved in 2012 by the FDA, based on a placebo-controlled, randomized withdrawal design study of 116 patients who were monitored for relapse symptoms. After randomized drug withdrawal, a majority of patients (75%) given placebo showed symptom relapse by two weeks compared to 9% of those patients who continued on Vyvanse (Brams M et al, J Clin Psychiatry 2012;73(7):977–983.)
Similar results were seen in a more recent study with 276 children; 16% of Vyvanse patients had symptom relapse compared to 68% of those on placebo (Coghill DR et al, J Am Acad Child Adolesc Psychiatry 2014;53(6):647–657). The fact that stimulant withdrawal leads to renewed symptoms is no shocker, though Shire Pharmaceuticals, which makes Vyvanse, gets kudos for being the first manufacturer to demonstrate this for all age groups.
How it Compares with Other ADHD Medications
So, Vyvanse is better than placebo, both in the short term and long term, but how do we rank it with other ADHD treatments?
All major published clinical trials of the drug have been funded by Shire, and there are no truly robust head-to-head comparator studies with competing stimulants. One crossover study of 6- to 12-year-old children had all 52 subjects start on Adderall XR at 10 mg/day, and doses were individualized to each patient’s optimal daily dose over a three-week period. Subjects then entered the double-blind crossover part of the study in which they received all three treatments sequentially (placebo, their optimized Adderall XR dose, an equivalent dose of Vyvanse) and the order of treatments was randomized. Patients improved on each of the stimulant medications when compared to placebo. However, there were not enough subjects to statistically compare the two active treatments, and we cynically wonder if this Shire-funded study was underpowered on purpose, so as to prevent a result that might have made Vyvanse look worse than Adderal (Biederman J et al, Biol Psychiatry 2007;62(9):970–976).
Another placebo-controlled study of Vyvanse in children, conducted in Europe, included an active reference arm of patients treated with Concerta. A total of 336 subjects were randomized to optimized dosing of Vyvanse (30, 50, or 70 mg/day), Concerta (18, 36, or 54 mg/day), or placebo for seven weeks. At the end of the study, 78% of Vyvanse subjects were deemed responders compared to 61% of Concerta subjects and 14% of placebo subjects. Similar to the Adderall XR study, this study was powered only to compare each of the two active drug groups to placebo, not to each other. Also of note, the maximum dose of Concerta is 54 mg/day in European countries compared to 72 mg/day in the US, which may have explained the lower response rate seen with that group (Coghill D et al, Eur Neuropsychopharmacol 2013;23(10):1208–1218).
There is one head-to-head study of Vyvanse compared to the non-stimulant noradrenergic atomoxetine (Strattera) in 267 children with previous inadequate response to methylphenidate (Dittmann RW et al, CNS Drugs 2013;27(12):1081–1092). Vyvanse out-performed Strattera, but nobody’s falling off their chair with these results, since other studies have established that Strattera is a less effective ADHD treatment than stimulants in general.
Deciding the Merits
Given the absence of well-designed studies comparing Vyvanse with other stimulants, how else are we to decide on its merits? Let’s focus on the two other long-acting amphetamine preparations: Dexedrine Spansules and Adderall XR. We can check Dexedrine Spansules off the list, because it is even more expensive than Vyvanse (about $26/day for the brand and about $10/day for the generic). The generic Adderall XR is only $1.50/day, versus Vyvanse at about $7/day.
They both have about the same duration of action (8–12 hours). The long-acting property of Vyvanse is due to its formulation as a prodrug, whereas Adderall XR is a bead-filled capsule that mimics twice daily dosing (50% of beads are immediate-release and 50% are delayed-release). The prodrug design of Vyvanse may decrease the potential for misuse or abuse compared to Adderall XR, which can be snorted or injected. However, there are no studies comparing the abuse liabilities of the two drugs.
Anecdotally, some psychiatrists in the field have told The Carlat Psychiatry Report (TCPR) that they prefer Vyvanse because they perceive it as being more tolerable, with a smoother onset and offset of effects than Adderall XR. Are anecdotal impressions worth choosing a drug that’s nearly five times the expense of a competitor? You’ll be the judge of that one.
By the way, Shire is actively pursuing more indications for Vyvanse. Though they recently halted its development as a treatment for depression after two failed late-stage clinical trials, they continue to seek approval for its use in binge eating disorder and plan studies for ADHD in the very young (4- to 5-year-olds).
Dr. Carlat’s Verdict: Vyvanse: Maybe a little less addictive, maybe a little more tolerable…but certainly much more expensive than Adderall XR and Concerta. We give Shire an A+ for marketing.
Related content:
Vyvanse for Sluggish Cognitive Tempo in ADHD

Newsletters
Please see our Terms and Conditions, Privacy Policy, Subscription Agreement, Use of Cookies, and Hardware/Software Requirements to view our website.
© 2025 Carlat Publishing, LLC and Affiliates, All Rights Reserved.


_-The-Breakthrough-Antipsychotic-That-Could-Change-Everything.jpg?1729528747)



